![]() EXPERT
EXPERT
Dr. Thomas Christopher Spalla, M.D.
Ear-Nose and Throat Doctor (ENT)
Dr. Thomas Christopher Spalla M.D. is a top Ear-Nose and Throat Doctor (ENT) in Detroit, . With a passion for the field and an unwavering commitment to their specialty, Dr. Thomas Christopher Spalla M.D. is an expert in changing the lives of their patients for the better. Through their designated cause and expertise in the field, Dr. Thomas Christopher Spalla M.D. is a prime example of a true leader in healthcare. As a leader and expert in their field, Dr. Thomas Christopher Spalla M.D. is passionate about enhancing patient quality of life. They embody the values of communication, safety, and trust when dealing directly with patients. In Detroit, MI, Dr. Thomas Christopher Spalla M.D. is a true asset to their field and dedicated to the profession of medicine.
Dr. Thomas Spalla, M.D.
- Detroit, MI
- Accepting new patients
No results found
Can swimmer's ears cause deafness?
A swimmer's ear can cause a loss of hearing if the canal becomes swollen shut or filled with debris from the infection. This hearing loss will completely recover once this infection READ MORE
A swimmer's ear can cause a loss of hearing if the canal becomes swollen shut or filled with debris from the infection. This hearing loss will completely recover once this infection resolves. In very rare circumstances, the infection, if untreated or if a severely immunocompromised state is present, could progress deeper into the skull. At this point, permanent hearing loss could result.
Lung issue?
Your asthma is a possibility in this situation, but I'd strongly consider nocturnal reflux (from your stomach) as a contributing factor. See your pulmonologist first and mention READ MORE
Your asthma is a possibility in this situation, but I'd strongly consider nocturnal reflux (from your stomach) as a contributing factor. See your pulmonologist first and mention this as a possibility. In the meantime, try elevating the head of your bed and make sure you don't eat (or drink much) for at least a couple hours before lying down at night (that includes lying down on the couch etc).
Parotid seroma keeps coming back after draining
This can be a very frustrating issue for both the patient and surgeon. It is typically self-limiting. First line therapy is recurrent aspiration of the fluid collection. The pocket READ MORE
This can be a very frustrating issue for both the patient and surgeon. It is typically self-limiting. First line therapy is recurrent aspiration of the fluid collection. The pocket typically gets smaller and scars itself off with time. Occasionally, a drain will be placed to help prevent the need for repeated visits and aspirations, but isn't without its own drawbacks.
If this occurs long-term, Botox (or similar agents) may be useful. Fortunately, I've never had it persist long enough to require such intervention.
I have used Botox for patients whom I've seen in consultation for Frey's syndrome (sweating over this area while eating) which can be a consequence of parotid surgery.
If this occurs long-term, Botox (or similar agents) may be useful. Fortunately, I've never had it persist long enough to require such intervention.
I have used Botox for patients whom I've seen in consultation for Frey's syndrome (sweating over this area while eating) which can be a consequence of parotid surgery.

Bad cough and lump in throat?
I do not feel that the lump you see in your throat is at all related to what you have been experiencing. The lump in question appears to be what we would describe as a solitary READ MORE
I do not feel that the lump you see in your throat is at all related to what you have been experiencing. The lump in question appears to be what we would describe as a solitary papilloma. You can think of this as a wart.
I would recommend the removal of this by an ear nose and throat doctor. This can easily be accomplished in the office under local anesthesia.
I would recommend the removal of this by an ear nose and throat doctor. This can easily be accomplished in the office under local anesthesia.

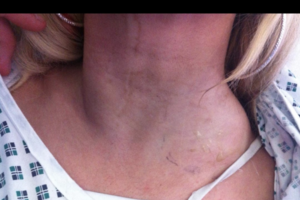
Why does my neck swell?
You definitely need to see an ENT physician for evaluation and management. This could represent a number of things.
When you go, let the physician know:
how long it's been READ MORE
You definitely need to see an ENT physician for evaluation and management. This could represent a number of things.
When you go, let the physician know:
how long it's been there;
any interventions for this;
any childhood cancers;
any history of radiation to the head and neck;
any family history of cancer;
any prior biopsies, imaging studies;
any change in: voice, swallowing, weight, cold/heat intolerance, other lumps noted, history of any thyroid issues, history of skin cancer, smoking/alcohol history, medication usage, coughing up any blood;
note if it fluctuates in size, if there's any pain;
imaging studies and a biopsy (typically a needle) are warranted.
When you go, let the physician know:
how long it's been there;
any interventions for this;
any childhood cancers;
any history of radiation to the head and neck;
any family history of cancer;
any prior biopsies, imaging studies;
any change in: voice, swallowing, weight, cold/heat intolerance, other lumps noted, history of any thyroid issues, history of skin cancer, smoking/alcohol history, medication usage, coughing up any blood;
note if it fluctuates in size, if there's any pain;
imaging studies and a biopsy (typically a needle) are warranted.

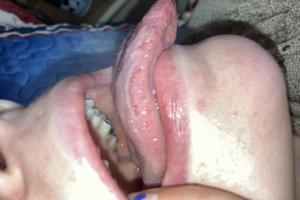
What is wrong with my tongue?
Overall, the condition would be considered to be glossitis which is inflammation of the tongue. People can also have a condition called geographic tongue which has a similar appearance. READ MORE
Overall, the condition would be considered to be glossitis which is inflammation of the tongue. People can also have a condition called geographic tongue which has a similar appearance. This may be associated with dietary, hormonal, and lifestyle changes. Additionally, certain medications and oral care products may result in this situation. This can often occur around the time of an illness such as a viral upper respiratory tract infection. It is typically self-limited. During these times, I recommend a soft bland diet, warm salt water gargles, and avoiding any oral care product with sodium lauryl sulfate.



What is the hissing sound I am hearing in my ears?
We would typically call this tinnitus. I would recommend that you see an ear nose and throat physician, as well as an audiologist for an ear and hearing examination as this, may READ MORE
We would typically call this tinnitus. I would recommend that you see an ear nose and throat physician, as well as an audiologist for an ear and hearing examination as this, may be able to determine a potential cause for this.
Something stuck in my mouth?
Check in with the doctor who pulled your tooth first. Even if it's unrelated to the tooth, they should be able to determine the cause. Given that it's under your tongue, it could READ MORE
Check in with the doctor who pulled your tooth first. Even if it's unrelated to the tooth, they should be able to determine the cause. Given that it's under your tongue, it could be a salivary stone. If this is the suspected cause, see an ENT doctor for assistance.
Nose tip cartilage lump?
It sounds like you are talking about your lower lateral cartilage. Take a look at some images on it and see if this correlates. There can be a lot of variability in its shape between, READ MORE
It sounds like you are talking about your lower lateral cartilage. Take a look at some images on it and see if this correlates. There can be a lot of variability in its shape between, and within, individuals.
Clogged right ear for 3 days?
You are experiencing Eustachian tube dysfunction. Try gentle autoinsufflation (pinching your nose closed and blowing) as this may help "pop" your ear. Without knowing your medical READ MORE
You are experiencing Eustachian tube dysfunction. Try gentle autoinsufflation (pinching your nose closed and blowing) as this may help "pop" your ear. Without knowing your medical history, I can't recommend specific medications. If this persists, see an ENT physician for assistance. Rest assured, this usually spontaneously resolves.
Foreign object in the ear canal?
It could be irritation from the foreign body or the manipulation to get it out. See an ENT physician for an examination and reassurance.
Sinus drainage?
Without knowing more about your medical history, medications, etc, it's safe to say:
-drink plenty of fluids
-use a humidifier/steam
-use a saline nasal flushing system
The READ MORE
Without knowing more about your medical history, medications, etc, it's safe to say:
-drink plenty of fluids
-use a humidifier/steam
-use a saline nasal flushing system
The very short-term/temporary (< 3 day) use of a nasal decongestant spray and a mucolytic (e.g., Mucinex) may be helpful, but run these by your primary care physician. Also, a nasal steroid spray can be helpful, but takes some time to reach maximal efficacy. Again, run this by your primary care physician.
-drink plenty of fluids
-use a humidifier/steam
-use a saline nasal flushing system
The very short-term/temporary (< 3 day) use of a nasal decongestant spray and a mucolytic (e.g., Mucinex) may be helpful, but run these by your primary care physician. Also, a nasal steroid spray can be helpful, but takes some time to reach maximal efficacy. Again, run this by your primary care physician.

How should I manage my symptoms?
You definitely need to seek in-person medical care as this could represent a whole host of different problems.
Why does my son wake up every morning with sneezing and a stuffy nose?
It certainly sounds allergic in nature. It may be time to have formal allergy testing done. Thoroughly clean his room. Get mattress and pillow dust mite covers. Keep pets out of READ MORE
It certainly sounds allergic in nature. It may be time to have formal allergy testing done. Thoroughly clean his room. Get mattress and pillow dust mite covers. Keep pets out of the room. Keep the humidity a little lower to minimize mold allergens.
Am I at a risk of throat cancer if I quit a smoking habit of 3 years?
Yes, you are still at an increased risk of cancer of the: mouth, throat, lungs, pancreas, kidneys, and bladder from years of smoking. That being said, quitting is the best thing READ MORE
Yes, you are still at an increased risk of cancer of the: mouth, throat, lungs, pancreas, kidneys, and bladder from years of smoking. That being said, quitting is the best thing you can do for yourself. See your physician if you have concerning symptoms.
Is it possible to experience nasal blockage after a slight blow to the nose?
It is possible to deviate the septum in such a manner. That being said, it's more likely just reactive mucosa swelling from the trauma. If your nose looks different externally, READ MORE
It is possible to deviate the septum in such a manner. That being said, it's more likely just reactive mucosa swelling from the trauma. If your nose looks different externally, see a nasal specialist ASAP for intervention. If the obstruction persists, you should seek medical attention.
Alzheimer's and loss of smell?
There is a well-established connection between Alzheimer's and a loss or decrease in sense of smell. In fact, that can be the first symptom in some patients. Of course, other underlying READ MORE
There is a well-established connection between Alzheimer's and a loss or decrease in sense of smell. In fact, that can be the first symptom in some patients. Of course, other underlying nasal pathologies may be contributing and should be ruled-out by an ENT doctor. Age-related neural degeneration can also play a role. This could be multifactorial.
Do I have tinnitus?
It does sound like you are experiencing what we would describe as tinnitus. There are many forms of tinnitus. During these times of acoustic trauma to the ear, it is important READ MORE
It does sound like you are experiencing what we would describe as tinnitus. There are many forms of tinnitus. During these times of acoustic trauma to the ear, it is important that you avoid any further injury to the ear with any type of loud sounds at all. Moving forward, I would recommend that you see an otolaryngologist for an examination as well as an audiologist for hearing testing. You can still use your headphones after this is fully evaluated by the doctors above. However, please keep your volume lower. The people sitting near you should not be able to hear your headphones as a good rule of thumb. Additionally, you should be able to hear somebody in the same room talk to you with a normal degree of loudness to their voice and you should still be able to hear them. These are good rules of thumb with regards to hearing and headphone usage.
My child has frequent ear infections. Why is this happening?
I know this is a long referenced answer for you, but it should clear some things up for you. The first thing, however, is to establish whether or not these are truly ear infections READ MORE
I know this is a long referenced answer for you, but it should clear some things up for you.
The first thing, however, is to establish whether or not these are truly ear infections or something else.
INCIDENCE IN CONTROLLED POPULATIONS
The number of AOM episodes gradually increases from birth until it reaches its maximum between 6 and 12 months of age, and is then seen to decrease followed by an increase between 4-5 years of age with a second peak, slightlyless prominent [6]. The average number of episodes decreased from 1.2 per child during the first year to 0.4 per child on reaching the seventh year (Table 2) [8, 9]. In Teele's series 62.4% of children had atleast one episoide of otitis at the end of the first year, and 17.3% had atleast three episodes. This percentage reached 83%, 91% and 93% at the third, fifth and seventh year, respectively. The Scandinavian series (Table 3) gave noticeablylower numbers compared to the above; according to Pukander [18] and Ingvarsson [19], about 30% of children had their first episode of AOM before the first birthday, and the maximum incidence occurred between the ages of 1 and 2 (57.3%). Aniansson [20] prospectively analyzed 400 Swedish children; within the first year, 21% had experienced atleast one episode of AOM and only 6% two or more. Of 111 episodes of infection, 6% took place within the first 2 months, 32% between 2 and 7 months, and 62% between 8 and 12 months. Kero [21] found one or more episodes of AOM in 34.5% in the first year, and three or more in only 9.7%. The average time for the first episode to occur was 8 months, and 25% of children had their first episode of AOM during the first 6 months. Finally, of the 1013 American infants monitored by Duncan [22] during their entire first year oflife, 476 (47%) had atleast one episode of otitis media, and 169 (17%) had recurrent otitis media defined as three or more episodes of AOM in a 6-month period or four episodes in 12 months. In our follow-up series of 144 Spanish infants monitored during their first year, 44% suffered from one or more episodes of AOM, and only 7.6% three or more. From the group of children suffering from otitis, 8.7% had their first episode before their third month, 26.3% between 3 and 6 months, 33.3% between 6 and 9 months, and 31.5% between 9 and 12 months.
Table 2AOM in 498 chddren in Greater Boston (from 6)
Year oflife Mean (range) No. of episodes Percentage of children experiencing no episodes
0 1 2 3 4 5 6
1 1.2 (0-6) 38 31 14 10 5 2 <1
2 1.1 (0-6) 41 29 20 6 2 1 1
3 0.7 (0-6) 59 24 10 3 2 1 <1
4 0.8 (0-7) 58 23 11 4 3 1 <1
5 0.7 (0-6) 58 26 9 4 2 1 <1
6 0.6 (0-5) 63 24 8 2 1 <1 0
7 0.4 (0-5) 70 20 6 2 1 <1 0
Open table in a new tab
Table 3Incidence of children with AOM in the first year oflife
First author Year of publication No. of episodes
1 2 3
Teele, 1989 62.0% – 17.3%
Aniansson, 1994 21.0% 6.0% –
Kero, 1987 34.5% – 9.7%
Duncan, 1993 64.0% 17.0%
Schwartz, 1990 46.0% – –
Pukander, 1982
Ingvarson, 1983 30.0% – –
Lundgren, 1983
Baraibar, 1994 44.0% 17.3% 7.8%
Open table in a new tab
Most children have only occasional episodes of AOM, or none at all, but some are subject to recurrent or severe ear infections; those children named ‘otitis-prone’ are defined as those having had three or more episodes of AOM before their third year [6]. The risk features for recurrent middle ear infections which clearly modified its incidence and epidemiology are analyzed below.
HOST FACTORS
Sex predominance
As with most infectious childhood diseases, AOM seems to occur significantly more often in males than in females. Different prospective series [21, 23, 24, 25] showed that the total number of episodes of AOM and the number of episodes of recurrent otitis media are indeed higher in male subjects, as well as the number of visits due to AOM and the number of performed myringotomies and tympanocenteses [1]. Teele [6] reported that 66% and 86% of males versus 53% and 77% of females had had an episode of otitis within their first year and third year respectively, and male gender was significantly associated with an increased risk of having atleast one episode of AOM and recurrent AOM in the period between the first and third years. According to Stenstrom's data, 61% of the otitis-prone group were male children and 35% female, while other series (including ours) could find no sex differences [20, 22]. The reasons for this sex predominance are not well known. The study of Matsuoka [26] is an attempt to describe the concentrations of three major inmunoglobulins and IgG2 in healthy children and ‘otitis-prone’ children from 2 to 7 years of age, divided into 1-year interval age/sex categories. The main effect of otitis- proneness was statistically significant for IgG2 and it was assumed that thelowlevel of IgG2 was one of the causes of the AOM's high frequency. Again, the main effect on the male gender was statistically significant for IgG2levels, so the authors concluded that this male-dominant tendency could be associated with an insufficient IgG2 antibody production, or a delayed maturation of IgG2 in male subjects. Nonetheless, more studies will be needed in order to confirm such results.
Ethnicity
The marked ethnic differences in the frequency of AOM are well documented [7] although, to some authors [6], it is not clear whether the ethnicity itself could be a factor which predisposes to otitis (or offers protection against it), or whether different ethnic groups have different access to medical care. There are other series where such differences are evident. Afro-American black children haveless episodes of otitis, attributed to anatomic differences in the eustachian tube structure [27], whereas, on the contrary, Inuits from Alaska and Canada, American Indian children (Apache) and Australian aborigines have a higher incidence, perhaps related to precociousness and type of their nose-pharynx colonization [28]. Caucasian children [9, 19, 23, 29] have an intermediate frequency.
Birth order
As a general rule, second-born children seem to be more prone to otitis than the first-born, presumably as a consequence of a greater and earlier exposure to respiratory infections delivered into the family by the other sibling. This is true even if the first-born does not have a history of acute or recurrent otitis. According to Wright's data [9], race and birth order seem to have certain effects on rates of otitis in childrenless than 2 years old; in white subjects, there was a significantlinear correlation between the incidence of otitis in the first and second child, which is particularly strong if the first child had frequent otitis. The same trends were present in black children, but because of thelower incidence they were not significant. These variations are attributable to both interfamilial and intrafamilial differences, with alower variance between family members than between unrelated children. Frederick [30] and Kero [21] also find a greater incidence of otitis among children who have siblings. Kero gives an ‘odds ratio’ of 2.5 to compare three or more versus 0 siblings, 2.0 in 1-2 versus 0, and 1.3 in three or more versus 1-2 siblings. Teele found, by means of a univariate analysis, that having a sibling was significantly associated with an increased risk for more than one episode of AOM, for recurrent AOM in the first year oflife and for recurrent AOM in the third year oflife. Whether this relationship has a genetic base or an environmental one is not clear, because sharing a room with a sibling was also significantly associated with one or more episodes of AOM and with having recurrent AOM during the first year oflife. Finally, Teele finds no relationship with the total number of siblings.
Early occurrence of infection
AOM is mainly a disease of early infancy. Children who have only had a few episodes (less than three) before their third birthday are not prone to have any more problems with AOM. The child's age when the first episode occurs seems to be among the most reliable predictors for recurrent AOM [6, 18], in such a way that the more precocious it is, the more recurrence will be experienced, and thelonger will be the period with middle ear effusion. Breast-fed babies who had their first AOM before the second month oflife had an average of 3.5 months of bilateral effusion compared with 1.2 months for those who startedlater [29]; this would indicate that children who areliable to suffer from early infections are equally unable to clear middle ear effusion, thus remaining particularlyliable to suffer from reinfections. Infections in early infancy mark an underlying disability; this ‘predisposition’ of the infant to AOM is probably caused by some features of the eustachian tube (ET) anatomy. The role of competence of the ET is dramatically illustrated by the incidence of otitis which occurs in patients with cleft palate or otherless severe defects in the anatomy—cleft uvula [31], submucous cleft—or in the musculature which controls the ostia of the eustachian tube, which are normally closed except while yawning and swallowing [32]. The variations in child-to-child ET anatomy (shorter, wider, more horizontal), nasopharynx dimensions [33] and physiology (incomplete versus complete development of the musculature governing tensor veli palatini) could explain the otitis frequency differences. In the same way, cranial anatomy (cranial base relationships) could also play a role.
During the first 6-12 months oflife, the neonatal immune system passes through a transitory state, from the protection afforded by passively acquired maternal antibody to the independent generation of a fully capable humoral, cellular and mucosal immune system. Infections which have started more easily during these first months (most of all in children exposed to additional risk factors, such as being taken to day-care centers or coming into contact with older siblings with infections) could damage the mucosal histology and alter the ET and the ciliary function, which would predispose to subsequent infections [9]. In 540 children who had experienced their first episode of AOM before the second year oflife and had been observed for atleast 24 months after this initial diagnosis [6], the age of the first AOM episode was inversely associated with the risk of having one or more, two or more and three or more additional diagnoses of AOM within the 12 months after the first diagnosis (Table 4).
Table 4Association of age at first episode of AOM and number of additional episodes of AOM during 24 months of observation, after first episode (from 6).
Age at first episode No. of children Percentage experiencing additional episodes of AOM
0 1 2
1-6 months 237 14 24 62
7-12 months 163 25 32 43
> 12 months 140 50 24 26
Open table in a new tab
Sibling history of severe or recurrent ear infections
Children who have AOM or recurrent otitis media have a greater probability of having younger siblings with otitis than other children who never experienced it. Whether this fact has a genetic basis or an environmental one is a much discussed matter, and it islikely that both factors arelinked.
In the Boston study [6], a sibling history of recurrent AOM was among the strongest predictors of recurrent AOM, but no one has statistically associated sharing a room with a sibling with AOM. The classical data on Apache children would support the greater importance of the genetic factor (adopted Apache children had more episodes of AOM than did their non-Apache siblings and had a rate of illness similar to that of Apache children who remained within the reservation'slimits) [1]. The response to polysaccharide antigens identifies differences between children with recurrent AOM and controls in markers of geneticloci involved in immune response to pneumococcal polysaccharides. Stenstrom [25] investigated the frequency of acute infectious disease and allergy in a group of 252 otitis-prone children prospectively followed (from 2 to 7 years), and compared them with a control group matched for age and sex. The otitis-prone children accounted for an average number of ambulatory visits of 38.8 versus 9.2 in the control group, had been hospitalized three or more times, had two to four times as many diagnoses of rhinopharyngitis, bacterial rhinitis, sinusitis and tonsillitis as controls, and also had significantly more diagnoses of bronchopulmonary, gastrointestinal and urinary tract infections. The number of children in the otitis-prone group with allergic diseases was twice that in the other group (37% versus 17%). It was concluded that otitis-prone children are moreliable to suffer from different acute infectious diseases, and that suchliability has indeed a genetic basis. Teele [6] also found one of the strongest correlations between a sibling with a history of AOM and a greater risk for the following items: more than one otitis episode and more than three per year (odds ratio 2.23 and 3.29) at age 3 (odds ratio: 3.9 versus 2.58), and more than three at age 7 (odds ratio: 2.79), always showing statistically significant differences.
The importance of the age when the first episode occurs, sex, race and family bonds in children with severe and recurrent otitis media, suggests a genetically determined basis for predisposition to middle ear infections [2]. Nevertheless, external and environmental factors which may affect any children, genetically prone or not, are increasingly important.
ENVIRONMENTAL FACTORS
Season
On the one hand, and according to some authors [34], there is indeed a greater incidence of AOM during winter months which would correspond to that of the VURTI; on the other hand, some others do not find any statistically significant seasonality [6], either for the number of otitis episodes or recurrence, or regarding the duration of the effusion. It seems prudent to note that whenever this correlation exists (more in winter, spring and autumn), it is muchless prominent than that of the VURTI [16].
Socio-economic status
The influence of socio-economic factors on the incidence of otitis is not clear from an epidemiologic viewpoint. It is evident that in thelower social classes there can be a greater incidence of chronic or relapsing infections of the upper respiratory tract; some of these infections will become complicated by middle ear infections because these people seldom go to the physician's consulting room forlight diseases. As viral and bacterial otitis has a high degree of spontaneous healing, many of those episodes may never be diagnosed. The higher social classes, with a greater concern for prompt medical care, will go to the physician's consulting room to resolve every episode of infection, and will reduce their risk of suffering AOM complicating VURTI. However, factors such as taking the children to day-care centers (something very common among all social classes) and thelengthening of the breast-feeding period (which is sometimes greater in thelower classes in Spain) may alter these results.
In Teele's series [6], the socio-economic status was not significantly associated with increased incidence or recurrences of AOM, but it was in Sassen's [35] and in Kero's [21].
Smoke and air pollutants
Smoke and air pollutants arelinked to acute respiratory infections, particularly during the first 2 years of age [36, 37, 38]; mothers who smoke double the risk [16]. In Owen's work [39], an increasing rate of cigarette consumption per day at home is significantly associated with an increase in the amount of effusion time of the otitis media. Etzel [40] demonstrated that high concentrations of serum cotinine were associated with an increased incidence of AOM and increased duration of middle ear effusion after an acute episode. The 87 children with a serum cotinine higher than 2.5 ng/mL, had a 38% higher rate of new episodes of otitis media with effusion (OME) during the first three years oflife than those children withlower or unnoticeable serum cotinine concentrations (incidence density ratio 1.38). The availability of a biochemical marker such as serum (urinary and salivary) cotinine has allowed documentation of passive exposure to tobacco smoke to become more reliable than that provided by the case history alone [1]. Teele [6] has also found that regular parental smoking was significantly associated with the risk of one or more episodes of AOM and with the duration of the middle ear effusion in the first year, yet not in subsequent years. It is estimated [40] that 8% of OME cases and 17.6% of the days on which OME is suffered may be attributable to the exposure to tobacco smoke.
Passive smoking of environmental pollutants is a well-known cause of structural and physiologic changes in the respiratory tree, capable of producing pulmonary dysfunction [41], upper andlower respiratory illness and death. Increased exposure to cooking fires multiplies by four the risk of catching respiratory illnesses and, perhaps, this would be more considered especially in developed countries or underdeveloped rural communities. It has been demonstrated that particles produced by indoor cooking with biomass fuels exceed by nearly 20 times those produced by two-pack-per-day smokers. To our knowledge, the effects of these environmental pollutants on the incidence of AOM in infancy has not yet been reported.
Breast-feeding
The effects of breast-feeding on the frequency and age of the first episode of otitis are well documented. Even though some studies have failed to demonstrate such an association, they were retrospective cohort studies or those involving a small population [42, 43]. Duncan [22] and Hardy [23] demonstrated that infants exclusively breast-fed for 4 or more months had half the average number of AOM episodes than did those who were not breast-fed at all, and 40%less than those infants whose diets were supplemented with other kinds of food before 4 months. The recurrent AOM rate in infants exclusively breast-fed for 6 months or more was 10%, and 20% in those infants breast-fed forless than 4 months. This protection was independent of the recognized risk factors considered. Aniansson [20], in an elegant prospective study designed to achieve continued follow-up of 400 Swedish children, also demonstrated that the number of AOM episodes waslower in the breast-fed children in each age group. The age of the first AOM episode was inversely related to the duration of breast-feeding, and no episodes were detected in 24 children who had been breast-fed for more than 10 months when compared with 111 accounted episodes in the remaining 376 children. The association between the duration of breast-feeding and decreased incidence and risk of recurrence of AOM has been documented by many other authors [21, 22, 23, 39].
Furthermore, contrary to reports from some authors [44], Aniansson demonstrated that the frequency of VURTI was significantlylower in breast-fed infants than in weaned ones in all age groups (1-3, 4-7, 8-12 months): 23%, 13% and 4% versus 28%, 40% and 53%, respectively. It is well known that VURTI often precedes AOM; human milk contains specific antibodies against respiratory tract viruses which could explain this protection.
The frequency and promptness of onset of otitis have also been related to the habit of propping the bottle in bed [39, 45]. The reflux with the introduction of respiratory flora into the middle ear space may be a particular problem if the infant is swallowing in the supine position, and even the suction of pacifiers, which are often used in bed when the infant islying down, has been related recently [46] to increased risk of recurrent otitis media in children who are taken to day-care centers. However, Paradise [32] demonstrated that children with cleft palate, especially predisposed to reflux episodes, who take feeding bottles containing human milk had fewer days of middle ear effusion than infants fed through devices containing formula, and therefore the protection conferred by breast milk predominated over the deleterious effect of the feeding position. In the Boston series, Teele [6] found that breast-feeding for as short a period as 3 months strongly correlated with decreased risk for AOM during the entire first year oflife, but he did not find inverse tendencies regarding a decreasing risk related to an increased time. Sassen [35], also studying the duration of the protection conferred by breast-feeding, noticed that the risk of AOM was significantly decreased until 4 months after breast-feeding had been discontinued; then, without the protective effect of breast-feeding, and in some months, the children approached the risklevel estimated for the group of children who were never breast-fed. Approximately 12 months afterwards the risk was virtually the same as if the child had never been breast-fed. The risk was also significantly dependent on the infant's number of siblings and on the socio-economic status. Saarinen [47] compared children exclusively breast-fed for 6 months or more with infants weaned before 2 months, and also found a relative risk of 3.3 for two or more episodes of AOM (6% versus 19%) and a relative risk of 4.3 for four or more episodes in children 12-36 months of age (6% versus 36%).
It seems evident that breast-feeding does indeed protect against AOM, and such protection is not due to the feeding position, but to the addition of several biochemical, immunologic, physiologic and behavioral factors which interact in a way that is still not well understood [48]. This kind of protection could be particularly important in underdeveloped countries where the period of exclusive breast-feeding is usuallylonger. In Sudan's children, Shaaban [49] has shown that the short duration of breast-feeding is a risk factor for otitis media: 83% of his patients experienced the first attack of AOM during infancy. Twenty-five per cent of children breast-fed forless than 6 months, suffered AOM compared with only 10% for a control group, breast-fed for 13.7 months.
Type of day care
Inserting infants intolarge day-care groups clearly increases the incidence of respiratory infections including otitis media, in comparison with those without siblings, or cared for at home [1, 30]. This association was expected because family size and day-care contact are known to influence the acquisition of the nasopharyngeal flora during the first year oflife, and because bacterial carriage was found to be associated with an increased risk for AOM. Everybody is in agreement with the fact that taking care of babies inlarge day-care centers is one of the strongest factors associated with transmission and amplification of viral and bacterial infectious diseases (AOM included), especially if we consider that many children in our country neither stay home instead of going to the day-care center when they are ill, nor follow the exclusion criteria recommended by experts [50]. Although in the Boston series [6], and according to the authors, day-care assistance was not carefully evaluated, the study identified more episodes of otitis among the children who go to day-care centers than in those cared for at home. The Wald study [51] was in fact undertaken to compare prospectively the frequency, nature and severity of respiratory infections in 244 children enrolled at birth and supervised from the twelfth to the eighteenth month (with 159 children cared for at home, 40 in group care, and 45 in day-care centers). The groups were comparable with each other by sex, ethnicity, number of siblings and family history of allergy. Children cared for at home had an average of 4.7 illnesses per year in contrast to 6.0 for children in group care and 7.1 for children in day-care centers. Like the number of episodes of severe disease the period of the illness duration was minimal in home-cared children, and maximal in the day-care group. Among the 2741 respiratory tract infections suffered by children in this cohort, 29% were complicated by otitis media and, during the child's first 2 years, this proportion was significantly higher among children attending group care and center care than among children in home care. Moreover, myringotomy and tube placement for recurrent or persistent otitis media were performed in 21% of the children in center care versus 3% of children in home care. Many other authors [8, 18, 21, 52, 53] confirm the strong correlation between day care, acute viral respiratory infection and increased incidence of AOM. Data from Strangert's study [54] demonstrate that 58% of the children whose age takes in the 6-11-month period, 41% of those between 12 and 17 months and 21% of those between 18 and 23 months received antibiotic treatment for one or more episodes of AOM during the 9 months itlasted. In comparing the types of child care, he observed that 37% of day-cared children experienced AOM (2.2 AOM episodes per child), vs. 43% in the family group-cared (2.0 AOM episodes per child) and 23% in the home-cared group (1.3 AOM episodes per child). The annual rates of otitis media were 1.2, 1.3 and 0.5 respectively. Stahlberg [55], in 257 Finnish children, and Reves [56] also found annual rates of 2.3, 0.9 and 1.1, and 6.0, 1.6 and 1.6 respectively. In thelast study, the relative risk of a visit to the physician for a new episode of upper respiratory tract infection, and for otitis media in children in day care versus home care, was 2.7 and 3.8 respectively. The percentage of children receiving antibiotics was 35.7% in day care and 8.2% in home care, and the number of days with antibiotic was also greater in the day-care group. Hardy [23] reported that 17% of the children younger than 6 years had had repeated ear infections in the year preceding their study; the factors significantly associated with repeated ear infections were age (1-2 years), ethnicity (white), sex (male) and a medical case history of repeated tonsillitis, enlarged adenoids or asthma. When these factors were controlled, children in current child care arrangements (settings with more than six children) still had a 50% higher chance of repeated ear infections than children cared for at home.
Collet [57] performed a prospective cohort study comparing the risk of recurrent infections in 1242 children attending: (a) family day care (three children orless), (b) small day care (10-20 children), and (c)large day-care centers (40 children or more), during an 8.5-month period of supervision. Compared with (a), (b) presented a higher risk for: six or more total infectious episodes (odds ratio: 2.6), five or more upper respiratory tract infections (odds ratio: 2.2), two or more episodes of otitis media (odds ratio: 2.6), two or more episodes of conjunctivitis (odds ratio: 2.1) and two or more episodes of croup (odds ratio: 4.1). The risk of (c) was surprisingly intermediate between (b) and (a). Finally, two of the most important epidemiologic studies performed to demonstrate the strong association between otitis and day-care assistance have been carried out in northern Europe. Tos [58] performed tympanometry on 80% of the 2-year-old childrenliving in Copenhagen and born during the first half of each month over the year: approximately 50% of the children were found to have an abnormal tympanogram at any given time during the study. Children attending day care were twice aslikely to have tympanometric evidence of otitis media. Fiellau-Nikolajsen [59] obtained the same results in 96% of the 3-year-olds in the Danish community.
Other factors
Some other factors have also been associated with an increased incidence of acute otitis media [60, 61]:
1.
The ‘bad-responding’ children, who suffer antibiotic failures despite adequate antibiotic treatment selected according to cultures; these children are usually the youngest (10.6 months versus 18.5 months) affected.
2.
Children with recurrent viral infections.
3.
Children who initiate illnesses in winter months, specially when they are, at that moment, their period oflesser immunologic competence (6-12 months old). The risk probabilities can increase if there are other associated factors, like beginning the day-care center assistance, having not been breastfed or having a sibling with recurrent AOM or VURTI.
4.
Careless parents who fail to administer the antibiotic treatment, or to provide or follow an adequate medical control.
5.
The child's own asthma, eczema or allergy [42, 43].
6.
Prematurity [21].
The factors not consistently associated with increased incidence of AOM are birth weight and sibling or parental history of asthma, eczema or allergy.
The first thing, however, is to establish whether or not these are truly ear infections or something else.
INCIDENCE IN CONTROLLED POPULATIONS
The number of AOM episodes gradually increases from birth until it reaches its maximum between 6 and 12 months of age, and is then seen to decrease followed by an increase between 4-5 years of age with a second peak, slightlyless prominent [6]. The average number of episodes decreased from 1.2 per child during the first year to 0.4 per child on reaching the seventh year (Table 2) [8, 9]. In Teele's series 62.4% of children had atleast one episoide of otitis at the end of the first year, and 17.3% had atleast three episodes. This percentage reached 83%, 91% and 93% at the third, fifth and seventh year, respectively. The Scandinavian series (Table 3) gave noticeablylower numbers compared to the above; according to Pukander [18] and Ingvarsson [19], about 30% of children had their first episode of AOM before the first birthday, and the maximum incidence occurred between the ages of 1 and 2 (57.3%). Aniansson [20] prospectively analyzed 400 Swedish children; within the first year, 21% had experienced atleast one episode of AOM and only 6% two or more. Of 111 episodes of infection, 6% took place within the first 2 months, 32% between 2 and 7 months, and 62% between 8 and 12 months. Kero [21] found one or more episodes of AOM in 34.5% in the first year, and three or more in only 9.7%. The average time for the first episode to occur was 8 months, and 25% of children had their first episode of AOM during the first 6 months. Finally, of the 1013 American infants monitored by Duncan [22] during their entire first year oflife, 476 (47%) had atleast one episode of otitis media, and 169 (17%) had recurrent otitis media defined as three or more episodes of AOM in a 6-month period or four episodes in 12 months. In our follow-up series of 144 Spanish infants monitored during their first year, 44% suffered from one or more episodes of AOM, and only 7.6% three or more. From the group of children suffering from otitis, 8.7% had their first episode before their third month, 26.3% between 3 and 6 months, 33.3% between 6 and 9 months, and 31.5% between 9 and 12 months.
Table 2AOM in 498 chddren in Greater Boston (from 6)
Year oflife Mean (range) No. of episodes Percentage of children experiencing no episodes
0 1 2 3 4 5 6
1 1.2 (0-6) 38 31 14 10 5 2 <1
2 1.1 (0-6) 41 29 20 6 2 1 1
3 0.7 (0-6) 59 24 10 3 2 1 <1
4 0.8 (0-7) 58 23 11 4 3 1 <1
5 0.7 (0-6) 58 26 9 4 2 1 <1
6 0.6 (0-5) 63 24 8 2 1 <1 0
7 0.4 (0-5) 70 20 6 2 1 <1 0
Open table in a new tab
Table 3Incidence of children with AOM in the first year oflife
First author Year of publication No. of episodes
1 2 3
Teele, 1989 62.0% – 17.3%
Aniansson, 1994 21.0% 6.0% –
Kero, 1987 34.5% – 9.7%
Duncan, 1993 64.0% 17.0%
Schwartz, 1990 46.0% – –
Pukander, 1982
Ingvarson, 1983 30.0% – –
Lundgren, 1983
Baraibar, 1994 44.0% 17.3% 7.8%
Open table in a new tab
Most children have only occasional episodes of AOM, or none at all, but some are subject to recurrent or severe ear infections; those children named ‘otitis-prone’ are defined as those having had three or more episodes of AOM before their third year [6]. The risk features for recurrent middle ear infections which clearly modified its incidence and epidemiology are analyzed below.
HOST FACTORS
Sex predominance
As with most infectious childhood diseases, AOM seems to occur significantly more often in males than in females. Different prospective series [21, 23, 24, 25] showed that the total number of episodes of AOM and the number of episodes of recurrent otitis media are indeed higher in male subjects, as well as the number of visits due to AOM and the number of performed myringotomies and tympanocenteses [1]. Teele [6] reported that 66% and 86% of males versus 53% and 77% of females had had an episode of otitis within their first year and third year respectively, and male gender was significantly associated with an increased risk of having atleast one episode of AOM and recurrent AOM in the period between the first and third years. According to Stenstrom's data, 61% of the otitis-prone group were male children and 35% female, while other series (including ours) could find no sex differences [20, 22]. The reasons for this sex predominance are not well known. The study of Matsuoka [26] is an attempt to describe the concentrations of three major inmunoglobulins and IgG2 in healthy children and ‘otitis-prone’ children from 2 to 7 years of age, divided into 1-year interval age/sex categories. The main effect of otitis- proneness was statistically significant for IgG2 and it was assumed that thelowlevel of IgG2 was one of the causes of the AOM's high frequency. Again, the main effect on the male gender was statistically significant for IgG2levels, so the authors concluded that this male-dominant tendency could be associated with an insufficient IgG2 antibody production, or a delayed maturation of IgG2 in male subjects. Nonetheless, more studies will be needed in order to confirm such results.
Ethnicity
The marked ethnic differences in the frequency of AOM are well documented [7] although, to some authors [6], it is not clear whether the ethnicity itself could be a factor which predisposes to otitis (or offers protection against it), or whether different ethnic groups have different access to medical care. There are other series where such differences are evident. Afro-American black children haveless episodes of otitis, attributed to anatomic differences in the eustachian tube structure [27], whereas, on the contrary, Inuits from Alaska and Canada, American Indian children (Apache) and Australian aborigines have a higher incidence, perhaps related to precociousness and type of their nose-pharynx colonization [28]. Caucasian children [9, 19, 23, 29] have an intermediate frequency.
Birth order
As a general rule, second-born children seem to be more prone to otitis than the first-born, presumably as a consequence of a greater and earlier exposure to respiratory infections delivered into the family by the other sibling. This is true even if the first-born does not have a history of acute or recurrent otitis. According to Wright's data [9], race and birth order seem to have certain effects on rates of otitis in childrenless than 2 years old; in white subjects, there was a significantlinear correlation between the incidence of otitis in the first and second child, which is particularly strong if the first child had frequent otitis. The same trends were present in black children, but because of thelower incidence they were not significant. These variations are attributable to both interfamilial and intrafamilial differences, with alower variance between family members than between unrelated children. Frederick [30] and Kero [21] also find a greater incidence of otitis among children who have siblings. Kero gives an ‘odds ratio’ of 2.5 to compare three or more versus 0 siblings, 2.0 in 1-2 versus 0, and 1.3 in three or more versus 1-2 siblings. Teele found, by means of a univariate analysis, that having a sibling was significantly associated with an increased risk for more than one episode of AOM, for recurrent AOM in the first year oflife and for recurrent AOM in the third year oflife. Whether this relationship has a genetic base or an environmental one is not clear, because sharing a room with a sibling was also significantly associated with one or more episodes of AOM and with having recurrent AOM during the first year oflife. Finally, Teele finds no relationship with the total number of siblings.
Early occurrence of infection
AOM is mainly a disease of early infancy. Children who have only had a few episodes (less than three) before their third birthday are not prone to have any more problems with AOM. The child's age when the first episode occurs seems to be among the most reliable predictors for recurrent AOM [6, 18], in such a way that the more precocious it is, the more recurrence will be experienced, and thelonger will be the period with middle ear effusion. Breast-fed babies who had their first AOM before the second month oflife had an average of 3.5 months of bilateral effusion compared with 1.2 months for those who startedlater [29]; this would indicate that children who areliable to suffer from early infections are equally unable to clear middle ear effusion, thus remaining particularlyliable to suffer from reinfections. Infections in early infancy mark an underlying disability; this ‘predisposition’ of the infant to AOM is probably caused by some features of the eustachian tube (ET) anatomy. The role of competence of the ET is dramatically illustrated by the incidence of otitis which occurs in patients with cleft palate or otherless severe defects in the anatomy—cleft uvula [31], submucous cleft—or in the musculature which controls the ostia of the eustachian tube, which are normally closed except while yawning and swallowing [32]. The variations in child-to-child ET anatomy (shorter, wider, more horizontal), nasopharynx dimensions [33] and physiology (incomplete versus complete development of the musculature governing tensor veli palatini) could explain the otitis frequency differences. In the same way, cranial anatomy (cranial base relationships) could also play a role.
During the first 6-12 months oflife, the neonatal immune system passes through a transitory state, from the protection afforded by passively acquired maternal antibody to the independent generation of a fully capable humoral, cellular and mucosal immune system. Infections which have started more easily during these first months (most of all in children exposed to additional risk factors, such as being taken to day-care centers or coming into contact with older siblings with infections) could damage the mucosal histology and alter the ET and the ciliary function, which would predispose to subsequent infections [9]. In 540 children who had experienced their first episode of AOM before the second year oflife and had been observed for atleast 24 months after this initial diagnosis [6], the age of the first AOM episode was inversely associated with the risk of having one or more, two or more and three or more additional diagnoses of AOM within the 12 months after the first diagnosis (Table 4).
Table 4Association of age at first episode of AOM and number of additional episodes of AOM during 24 months of observation, after first episode (from 6).
Age at first episode No. of children Percentage experiencing additional episodes of AOM
0 1 2
1-6 months 237 14 24 62
7-12 months 163 25 32 43
> 12 months 140 50 24 26
Open table in a new tab
Sibling history of severe or recurrent ear infections
Children who have AOM or recurrent otitis media have a greater probability of having younger siblings with otitis than other children who never experienced it. Whether this fact has a genetic basis or an environmental one is a much discussed matter, and it islikely that both factors arelinked.
In the Boston study [6], a sibling history of recurrent AOM was among the strongest predictors of recurrent AOM, but no one has statistically associated sharing a room with a sibling with AOM. The classical data on Apache children would support the greater importance of the genetic factor (adopted Apache children had more episodes of AOM than did their non-Apache siblings and had a rate of illness similar to that of Apache children who remained within the reservation'slimits) [1]. The response to polysaccharide antigens identifies differences between children with recurrent AOM and controls in markers of geneticloci involved in immune response to pneumococcal polysaccharides. Stenstrom [25] investigated the frequency of acute infectious disease and allergy in a group of 252 otitis-prone children prospectively followed (from 2 to 7 years), and compared them with a control group matched for age and sex. The otitis-prone children accounted for an average number of ambulatory visits of 38.8 versus 9.2 in the control group, had been hospitalized three or more times, had two to four times as many diagnoses of rhinopharyngitis, bacterial rhinitis, sinusitis and tonsillitis as controls, and also had significantly more diagnoses of bronchopulmonary, gastrointestinal and urinary tract infections. The number of children in the otitis-prone group with allergic diseases was twice that in the other group (37% versus 17%). It was concluded that otitis-prone children are moreliable to suffer from different acute infectious diseases, and that suchliability has indeed a genetic basis. Teele [6] also found one of the strongest correlations between a sibling with a history of AOM and a greater risk for the following items: more than one otitis episode and more than three per year (odds ratio 2.23 and 3.29) at age 3 (odds ratio: 3.9 versus 2.58), and more than three at age 7 (odds ratio: 2.79), always showing statistically significant differences.
The importance of the age when the first episode occurs, sex, race and family bonds in children with severe and recurrent otitis media, suggests a genetically determined basis for predisposition to middle ear infections [2]. Nevertheless, external and environmental factors which may affect any children, genetically prone or not, are increasingly important.
ENVIRONMENTAL FACTORS
Season
On the one hand, and according to some authors [34], there is indeed a greater incidence of AOM during winter months which would correspond to that of the VURTI; on the other hand, some others do not find any statistically significant seasonality [6], either for the number of otitis episodes or recurrence, or regarding the duration of the effusion. It seems prudent to note that whenever this correlation exists (more in winter, spring and autumn), it is muchless prominent than that of the VURTI [16].
Socio-economic status
The influence of socio-economic factors on the incidence of otitis is not clear from an epidemiologic viewpoint. It is evident that in thelower social classes there can be a greater incidence of chronic or relapsing infections of the upper respiratory tract; some of these infections will become complicated by middle ear infections because these people seldom go to the physician's consulting room forlight diseases. As viral and bacterial otitis has a high degree of spontaneous healing, many of those episodes may never be diagnosed. The higher social classes, with a greater concern for prompt medical care, will go to the physician's consulting room to resolve every episode of infection, and will reduce their risk of suffering AOM complicating VURTI. However, factors such as taking the children to day-care centers (something very common among all social classes) and thelengthening of the breast-feeding period (which is sometimes greater in thelower classes in Spain) may alter these results.
In Teele's series [6], the socio-economic status was not significantly associated with increased incidence or recurrences of AOM, but it was in Sassen's [35] and in Kero's [21].
Smoke and air pollutants
Smoke and air pollutants arelinked to acute respiratory infections, particularly during the first 2 years of age [36, 37, 38]; mothers who smoke double the risk [16]. In Owen's work [39], an increasing rate of cigarette consumption per day at home is significantly associated with an increase in the amount of effusion time of the otitis media. Etzel [40] demonstrated that high concentrations of serum cotinine were associated with an increased incidence of AOM and increased duration of middle ear effusion after an acute episode. The 87 children with a serum cotinine higher than 2.5 ng/mL, had a 38% higher rate of new episodes of otitis media with effusion (OME) during the first three years oflife than those children withlower or unnoticeable serum cotinine concentrations (incidence density ratio 1.38). The availability of a biochemical marker such as serum (urinary and salivary) cotinine has allowed documentation of passive exposure to tobacco smoke to become more reliable than that provided by the case history alone [1]. Teele [6] has also found that regular parental smoking was significantly associated with the risk of one or more episodes of AOM and with the duration of the middle ear effusion in the first year, yet not in subsequent years. It is estimated [40] that 8% of OME cases and 17.6% of the days on which OME is suffered may be attributable to the exposure to tobacco smoke.
Passive smoking of environmental pollutants is a well-known cause of structural and physiologic changes in the respiratory tree, capable of producing pulmonary dysfunction [41], upper andlower respiratory illness and death. Increased exposure to cooking fires multiplies by four the risk of catching respiratory illnesses and, perhaps, this would be more considered especially in developed countries or underdeveloped rural communities. It has been demonstrated that particles produced by indoor cooking with biomass fuels exceed by nearly 20 times those produced by two-pack-per-day smokers. To our knowledge, the effects of these environmental pollutants on the incidence of AOM in infancy has not yet been reported.
Breast-feeding
The effects of breast-feeding on the frequency and age of the first episode of otitis are well documented. Even though some studies have failed to demonstrate such an association, they were retrospective cohort studies or those involving a small population [42, 43]. Duncan [22] and Hardy [23] demonstrated that infants exclusively breast-fed for 4 or more months had half the average number of AOM episodes than did those who were not breast-fed at all, and 40%less than those infants whose diets were supplemented with other kinds of food before 4 months. The recurrent AOM rate in infants exclusively breast-fed for 6 months or more was 10%, and 20% in those infants breast-fed forless than 4 months. This protection was independent of the recognized risk factors considered. Aniansson [20], in an elegant prospective study designed to achieve continued follow-up of 400 Swedish children, also demonstrated that the number of AOM episodes waslower in the breast-fed children in each age group. The age of the first AOM episode was inversely related to the duration of breast-feeding, and no episodes were detected in 24 children who had been breast-fed for more than 10 months when compared with 111 accounted episodes in the remaining 376 children. The association between the duration of breast-feeding and decreased incidence and risk of recurrence of AOM has been documented by many other authors [21, 22, 23, 39].
Furthermore, contrary to reports from some authors [44], Aniansson demonstrated that the frequency of VURTI was significantlylower in breast-fed infants than in weaned ones in all age groups (1-3, 4-7, 8-12 months): 23%, 13% and 4% versus 28%, 40% and 53%, respectively. It is well known that VURTI often precedes AOM; human milk contains specific antibodies against respiratory tract viruses which could explain this protection.
The frequency and promptness of onset of otitis have also been related to the habit of propping the bottle in bed [39, 45]. The reflux with the introduction of respiratory flora into the middle ear space may be a particular problem if the infant is swallowing in the supine position, and even the suction of pacifiers, which are often used in bed when the infant islying down, has been related recently [46] to increased risk of recurrent otitis media in children who are taken to day-care centers. However, Paradise [32] demonstrated that children with cleft palate, especially predisposed to reflux episodes, who take feeding bottles containing human milk had fewer days of middle ear effusion than infants fed through devices containing formula, and therefore the protection conferred by breast milk predominated over the deleterious effect of the feeding position. In the Boston series, Teele [6] found that breast-feeding for as short a period as 3 months strongly correlated with decreased risk for AOM during the entire first year oflife, but he did not find inverse tendencies regarding a decreasing risk related to an increased time. Sassen [35], also studying the duration of the protection conferred by breast-feeding, noticed that the risk of AOM was significantly decreased until 4 months after breast-feeding had been discontinued; then, without the protective effect of breast-feeding, and in some months, the children approached the risklevel estimated for the group of children who were never breast-fed. Approximately 12 months afterwards the risk was virtually the same as if the child had never been breast-fed. The risk was also significantly dependent on the infant's number of siblings and on the socio-economic status. Saarinen [47] compared children exclusively breast-fed for 6 months or more with infants weaned before 2 months, and also found a relative risk of 3.3 for two or more episodes of AOM (6% versus 19%) and a relative risk of 4.3 for four or more episodes in children 12-36 months of age (6% versus 36%).
It seems evident that breast-feeding does indeed protect against AOM, and such protection is not due to the feeding position, but to the addition of several biochemical, immunologic, physiologic and behavioral factors which interact in a way that is still not well understood [48]. This kind of protection could be particularly important in underdeveloped countries where the period of exclusive breast-feeding is usuallylonger. In Sudan's children, Shaaban [49] has shown that the short duration of breast-feeding is a risk factor for otitis media: 83% of his patients experienced the first attack of AOM during infancy. Twenty-five per cent of children breast-fed forless than 6 months, suffered AOM compared with only 10% for a control group, breast-fed for 13.7 months.
Type of day care
Inserting infants intolarge day-care groups clearly increases the incidence of respiratory infections including otitis media, in comparison with those without siblings, or cared for at home [1, 30]. This association was expected because family size and day-care contact are known to influence the acquisition of the nasopharyngeal flora during the first year oflife, and because bacterial carriage was found to be associated with an increased risk for AOM. Everybody is in agreement with the fact that taking care of babies inlarge day-care centers is one of the strongest factors associated with transmission and amplification of viral and bacterial infectious diseases (AOM included), especially if we consider that many children in our country neither stay home instead of going to the day-care center when they are ill, nor follow the exclusion criteria recommended by experts [50]. Although in the Boston series [6], and according to the authors, day-care assistance was not carefully evaluated, the study identified more episodes of otitis among the children who go to day-care centers than in those cared for at home. The Wald study [51] was in fact undertaken to compare prospectively the frequency, nature and severity of respiratory infections in 244 children enrolled at birth and supervised from the twelfth to the eighteenth month (with 159 children cared for at home, 40 in group care, and 45 in day-care centers). The groups were comparable with each other by sex, ethnicity, number of siblings and family history of allergy. Children cared for at home had an average of 4.7 illnesses per year in contrast to 6.0 for children in group care and 7.1 for children in day-care centers. Like the number of episodes of severe disease the period of the illness duration was minimal in home-cared children, and maximal in the day-care group. Among the 2741 respiratory tract infections suffered by children in this cohort, 29% were complicated by otitis media and, during the child's first 2 years, this proportion was significantly higher among children attending group care and center care than among children in home care. Moreover, myringotomy and tube placement for recurrent or persistent otitis media were performed in 21% of the children in center care versus 3% of children in home care. Many other authors [8, 18, 21, 52, 53] confirm the strong correlation between day care, acute viral respiratory infection and increased incidence of AOM. Data from Strangert's study [54] demonstrate that 58% of the children whose age takes in the 6-11-month period, 41% of those between 12 and 17 months and 21% of those between 18 and 23 months received antibiotic treatment for one or more episodes of AOM during the 9 months itlasted. In comparing the types of child care, he observed that 37% of day-cared children experienced AOM (2.2 AOM episodes per child), vs. 43% in the family group-cared (2.0 AOM episodes per child) and 23% in the home-cared group (1.3 AOM episodes per child). The annual rates of otitis media were 1.2, 1.3 and 0.5 respectively. Stahlberg [55], in 257 Finnish children, and Reves [56] also found annual rates of 2.3, 0.9 and 1.1, and 6.0, 1.6 and 1.6 respectively. In thelast study, the relative risk of a visit to the physician for a new episode of upper respiratory tract infection, and for otitis media in children in day care versus home care, was 2.7 and 3.8 respectively. The percentage of children receiving antibiotics was 35.7% in day care and 8.2% in home care, and the number of days with antibiotic was also greater in the day-care group. Hardy [23] reported that 17% of the children younger than 6 years had had repeated ear infections in the year preceding their study; the factors significantly associated with repeated ear infections were age (1-2 years), ethnicity (white), sex (male) and a medical case history of repeated tonsillitis, enlarged adenoids or asthma. When these factors were controlled, children in current child care arrangements (settings with more than six children) still had a 50% higher chance of repeated ear infections than children cared for at home.
Collet [57] performed a prospective cohort study comparing the risk of recurrent infections in 1242 children attending: (a) family day care (three children orless), (b) small day care (10-20 children), and (c)large day-care centers (40 children or more), during an 8.5-month period of supervision. Compared with (a), (b) presented a higher risk for: six or more total infectious episodes (odds ratio: 2.6), five or more upper respiratory tract infections (odds ratio: 2.2), two or more episodes of otitis media (odds ratio: 2.6), two or more episodes of conjunctivitis (odds ratio: 2.1) and two or more episodes of croup (odds ratio: 4.1). The risk of (c) was surprisingly intermediate between (b) and (a). Finally, two of the most important epidemiologic studies performed to demonstrate the strong association between otitis and day-care assistance have been carried out in northern Europe. Tos [58] performed tympanometry on 80% of the 2-year-old childrenliving in Copenhagen and born during the first half of each month over the year: approximately 50% of the children were found to have an abnormal tympanogram at any given time during the study. Children attending day care were twice aslikely to have tympanometric evidence of otitis media. Fiellau-Nikolajsen [59] obtained the same results in 96% of the 3-year-olds in the Danish community.
Other factors
Some other factors have also been associated with an increased incidence of acute otitis media [60, 61]:
1.
The ‘bad-responding’ children, who suffer antibiotic failures despite adequate antibiotic treatment selected according to cultures; these children are usually the youngest (10.6 months versus 18.5 months) affected.
2.
Children with recurrent viral infections.
3.
Children who initiate illnesses in winter months, specially when they are, at that moment, their period oflesser immunologic competence (6-12 months old). The risk probabilities can increase if there are other associated factors, like beginning the day-care center assistance, having not been breastfed or having a sibling with recurrent AOM or VURTI.
4.
Careless parents who fail to administer the antibiotic treatment, or to provide or follow an adequate medical control.
5.
The child's own asthma, eczema or allergy [42, 43].
6.
Prematurity [21].
The factors not consistently associated with increased incidence of AOM are birth weight and sibling or parental history of asthma, eczema or allergy.
A cough for almost 12 days?
You could have ruptured a small blood vessel with the coughing that you've been doing. Given that you see blood, I would strongly recommend you start by seeing your primary care READ MORE
You could have ruptured a small blood vessel with the coughing that you've been doing. Given that you see blood, I would strongly recommend you start by seeing your primary care physician for an examination. This will include listening to your lungs. A CXR or other imaging modality may be warranted depending on additional information.


